Copyright Cleanroom Technology. Reprinted with permission. Original article can be found Here.
by John Klostermyer, STERIS VHP Application Project Manager
Vaporised hydrogen peroxide biodecontamination systems can be installed and integrated into a plethora of cleanrooms or suits. Here John Klostermyer, VHP Application Project Manager at STERIS, provides some tips for drafting a process-oriented User Requirement Specification.

A portable VHP unit
Drug and device manufacturers are increasing their use of VHP (vaporised hydrogen peroxide) to treat individual cleanrooms and cleanroom suits. VHP generators are installed and integrated with common air handling components and building automation controls. This creates a utility-like, facility-wide, biodecontamination system where cycles can be initiated with the click of a mouse.
Integrated VHP systems involve more effort to install compared to portable systems, yet very little effort to operate. A frequently operated integrated system can save thousands of hours in labour over its operational life and having a plan for the VHP facility integration process is a key component to its success.
This is a short review of the basic types of VHP systems along with some of the key aspects that one should consider in creating a User Requirement Specification (URS) for this system.
Unique processes
Each VHP application supports a unique target enclosure being decontaminated. The spatial and environmental conditions between enclosures can vary widely. Some cleanrooms have ducting, others have an array of fan filter units and an open plenum. There are cleanrooms that include higher grade environments such as Isolators, RABS, laminar airflow workbenches and biosafety cabinets, all of which can be treated with VHP. Another common application is the rapid biodecontamination of materials being shuttled through material airlocks.
"A frequently operated integrated system can save thousands of hours in labour"
To address this wide range of processes, VHP equipment is generally divided into two different categories; integrated and portable units. They vary in how VHP is injected, contained, evenly distributed and purged from an enclosure. For example, if the process demands frequent biodecontamination, an integrated VHP system would be the best choice. Integrated systems minimise set-up time and effort, and offer the fastest cycle times. They also provide a single pass source of VHP to decontaminate one or multiple zones automatically without the use of manually placed fans.
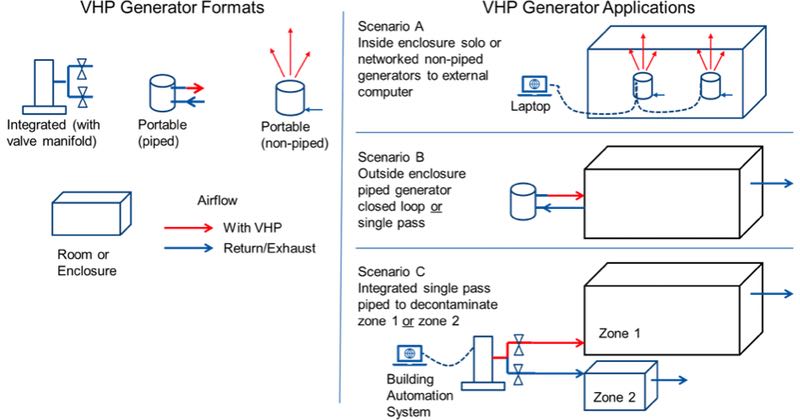
Figure 1: Types of VHP equipment
Non-piped portable VHP generators require no permanent installation and are always deployed inside a room or suite, alone or networked with others. If the rooms have a complex layout or are filled with equipment, fans may need to be positioned to enhance the distribution. Non-piped VHP generators typically have longer redeployment times, as they remain in a room during the aeration phase of the biodecontamination cycle. Thus, they are often best suited for less frequent use.
"Some cleanrooms have ducting, others have fan filter units and an open plenum"
Piped VHP portable generators are typically placed outside the room and VHP is piped in through a port. The lower airflow of a piped generator increases the need for well-placed fans. However, unlike the non-piped VHP generator, it can be redeployed to decontaminate another enclosure as the first one aerates.
VHP distribution
Air is the medium which transports VHP from the generator to all surfaces within the target enclosure. Its temperature, volume and humidity determine VHP concentration, distribution and physical state. Condensation along this pathway should be avoided. Hydrogen peroxide remains as a vapour with proper temperature and relative humidity control and flows with the air streams designed into the application.
This distribution process can be broken down into two categories:
Primary distribution: The point where liquid peroxide is vaporised and moved at a high concentration, typically through an insulated polymer pipe.
- For a portable non-piped generator the primary distribution is in the generator itself. An internal blower will disperse the VHP in the surrounding environment.
- Piped generators can be designed to deliver VHP into and adjacent room or enclosure or one that is hundreds of feet away. Larger applications use manifolds and an array of insulated polymer pipes to deliver the highly concentrated VHP to the target enclosure.
Secondary distribution: The point when highly concentrated VHP is diluted into the cooler air inside the enclosure and dispersed to decontaminate all exposed surfaces. Secondary distribution can be achieved with the use of well-positioned injection ports in a room or use the target area HVAC system. Manually placed fans are a less desirable, although a viable option.
It is useful if the footprint of the HVAC system matches that of the VHP target zone. Both single pass and recirculating HVAC systems can be used as a means of secondary distribution and aeration.
The URS
All the components used to produce, distribute, contain and eliminate VHP are part of a system. The system must operate in a reproducible manner to successfully and safely execute the biodecontamination process in accordance with a site-specific fumigation management plan. The sole purpose of the biodecontamination process is to support the primary work process in the facility.
"The HVAC system always plays a critical role in the secondary distribution of VHP"
A critical step to integrating a VHP system into a work process is to develop a URS that identifies the key requirements for the process. Creating a workable URS involves some planning but it will save time, resources and reduce project and process risk. A process-oriented URS focuses on production or process success factors such as turnover time, process efficacy, consistency, cost, safety etc.
A proactive process may contain many items to consider:
Zones and frequency - List the facility's individual "target" zones, their volume, the expected frequency of VHP biodecontamination and the acceptable cycle time that will support planned operations. Additional requirements here might include treating different rooms/zones in close sequence, or in groups. Table 1 gives examples of these for different types of rooms.
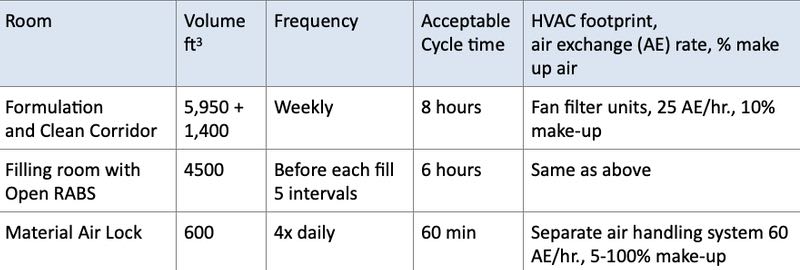
Table 1: Example zones and frequency of VHP decontamination for different rooms
Floor plan - Include a floor plan that identifies the perimeters of the target rooms. Indicate if groups of rooms should be treated at once and provide the room classification. Mark acceptable locations where VHP equipment can be deployed. Note that only portable, non-piped units are positioned inside the target area. If the groups of rooms are not adjacent to one another show a plan view indicating their proximity. It is best to control airflow where the footprint of the HVAC and the target zone align. If only the floor area is shown, add ceiling height or room volume. Then identify any specific enclosures within the space that may need individual biodecontamination such as the incubators shown in the example in figure 2.
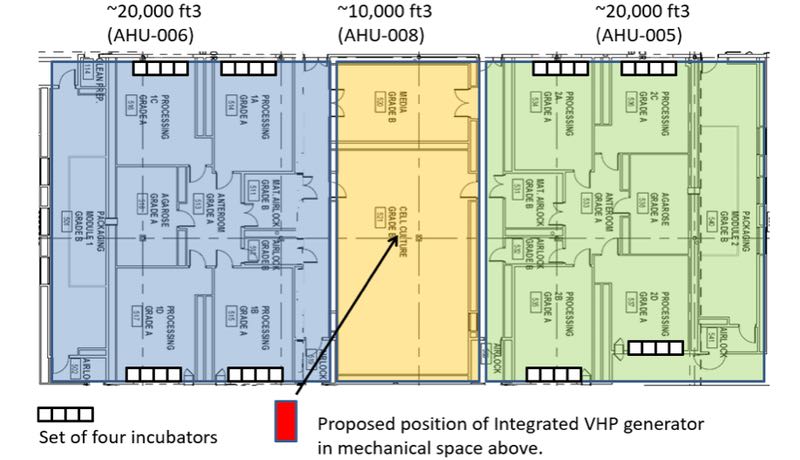
Figure 2: Floorplan showing potential target zones
HVAC - The HVAC system always plays a critical role in the secondary distribution of VHP and/or aeration. Provide a description of the HVAC system including air exchanges/hour, airflow rates, maximum per cent make up air and perhaps most importantly the coverage or footprint as it relates to the target zone. Include an HVAC schematic/P&ID and identify if it is single pass or recirculating, any drying systems, noise reducers or other components in line with the system. Provide materials of construction for the HVAC system, ducting and the verify its alignment with the VHP target zone.
Sequencing and redundancy - Consider the utilisation of the VHP equipment. For example, a generator can be used to decontaminate a material airlock or pass through during working hours and a cleanroom suite overnight. Determine if it makes sense to include redundancy in the system. For example, a backup VHP generator can be attached to a common manifold to serve multiple rooms or provide the flexibility to decontaminate multiple spaces at the same time.
Safety requirements - Safety considerations should be clearly outlined in a URS. This may include hydrogen peroxide concentration sensors within the target area and monitors in adjacent spaces. Also consider other safety mechanisms, such as automatic door locks, warning lights, E-Stops, alarms for differential pressure monitoring, and limit switches to verify valve positions on a distribution manifold. Additional safety measures can be found in the fumigation management plan.
Regulatory Requirements - What measures are needed for compliance with regulatory requirements? Is there a requirement for CFR 21-Part 11 compliance, including user administration, audit trail, print, alarm and trend logs? Identify local regulatory compliance items such a UL or CE marking. Consult the label for additional application-specific requirements.
Consumables - Is the consumable registered with the EPA or the EU BPR? Do lot numbers and expiration dates need to be automatically transferred into an internal data management system? What is the anticipated consumable consumption? What storage provisions need to be made?

STERIS' VHP M1000-T4, a pharma-grade VHP system for those requiring integration into an AHS/HVAC system
Documentation - What types of documentation should be offered with the equipment? Consider the user manual, P&ID, Electrical schematics, functional specification, spare parts list, controls and interface documentation. An SOP of each step of biodecontamination process should include aspects to comply with site safety, and local ordinances, the product label and applicable Agency oversight (i.e. FDA, CDC).
Control integration and utilities - Identify the type of control system the VHP system will integrate with. Include the communication protocol (i.e. Discrete I/O, Ethernet IP, BacNet, Profinet, Modbus, etc.) and a network diagram of the system. Include the critical items and process variables that need to be communicated between the systems. Identify what electrical utilities are available in the facility including voltage, phase and amperage.
Services - Which service support will be required to implement and maintain the process? Some may not apply depending on application. Pre-delivery this may include: Material Compatability/product testing, Design and mechanical integration support, control integration, application proposal, and a factory acceptance test. Post-delivery services may include: Installation/control integration, site acceptance test/installation/OQ, engineering runs/cycle development, operator training, and maintenance.
VHP is a highly effective antimicrobial with widespread use for treatment of critical environments. Each application is unique and different types of VHP equipment have been developed to support the various application needs. Implementing a successful biodecontamination solution goes beyond acquiring a piece of equipment.
When considering VHP implementation, drafting a process-oriented URS is a critical starting point. For successful final performance, the scope of developing a URS for any VHP system is a cross-functional effort involving the generator vendor, facility owner, the process owner and support groups such as validation, EH&S and local contractors as needed.
View STERIS VHP Sterilization & Biodecontamination Equipment